Motivating Case Studies
This ultrastructural microanatomy project aims to describe and better define the state of cells, organelles and supramolecular complexes as neurodegeneration begins and progresses in AD. Our intent is to seed, catalyze, and inform existing or follow-on hypothesis driven studies (by us and the biomedical research community). Therefore, we have focused our imaging and segmentation work to address questions regarding cell and network vulnerability – extending knowledge of the progression of dystrophic axons, cell bodies, dendrites and synaptic loss in AD. Motivated by current literature, this work will include assessment of targets that may yield structure/function insights into Ca2+ dysregulation, microtubule status/disruption, and AD associated changes to cell nuclei, chromatin, nuclear pore structure, and mitochondria.
Table 1: Initial targets for quantitative 3D EM imaging and analysis:
| Progression of Soma/Dendritic Degeneration |
Progression of Axonal Dystrophy at Perivascular Plaques |
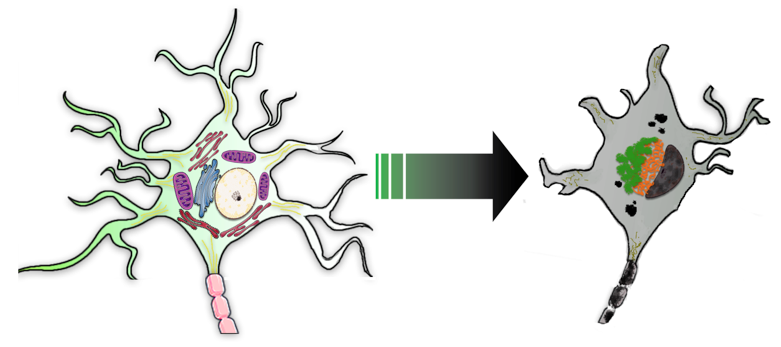
Legend for neuron and key organelles (normal cell on left) as altered during AD-associated degeneration (grey cell on right): orange = PHF; green = lipofuscin; Golgi and mitochondria are not perturbed in normal-appearing cell on left.
|
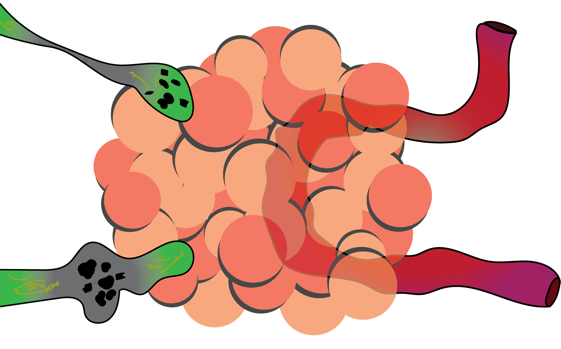
Legend: green = dystrophic axons; red = blood vessel; orange = perivascular plaque. Masliah, Ellisman and co-workers showed abnormal neurites localized to amyloid plaque interactions. This drawing is redrawn and simplified from that publication.
|
- Produce Quantitative Ultrastructural Anatomy:
- 1. Repeat and extend prior work reconstructing whole neurons and glia to determine volume fraction of PHF vs. cytoplasm (as functional definition to classify cells in early or late stages of AD associated decline).
- 2. Produce morphometrics of organelle systems and supramolecular complexes to quantify ultrastructure modifications relative to normal:
- • Disruption/modification of all internal membrane-bounded structures including analysis of:
- - The perinuclear organization of Golgi apparatus and the nuclear envelope (NE);
- - Changes in the location and volume fraction of cytoplasm occupied by endoplasmic reticulum;
- • Changes in mitochondrial structure and volume;
- • Cytoskeletal changes – conversion from microtubule bearing cytoskeleton to intermediate filaments and tau-bearing PHF’s, including their links to the NE;
- • Nuclei, chromatin, and nuclear pore structural changes.
|
- Produce Quantitative Ultrastructural Anatomy:
- 1. Reconstruct segments of neurites in close proximity to perivascular (amyloid) plaque material and proximally towards the cell soma (away from amyloid).
- 2. Produce morphometrics to quantify ultrastructure modifications relative to normal:
- • Evidence of retrograde transport blockages;
- • Microtubule disruption;
- • Mitochondrial changes;
- • Axoplasmic reticulum (Ca2+-sequestering SER of the axon);
- • Anterograde and retrograde axonal transport “vectors” (vesicles, vesiculo-tubular structures, or larger multi-lamellar, multi-vesicular or dense core structures).
|
* Masliah E, Mallory M, Deerinck T, DeTeresa R, Lamont S, Miller A, Terry RD, Carragher B, Ellisman M. (1993) Re-evaluation of the structural organization of neuritic plaques in Alzheimer's disease. J Neuropathol Exp Neurol. 1993 Nov;52(6):619-32.

